2020 Annual Review – Market Correction
Six months ago, I posted that the First Half of 2020 showed that the PIV Market was correcting itself. The FDA PIV List showed that fewer products receiving their first PIV certifications had multiple filers. This seemed to be a natural market correction — over the past few years, the PIV Market saw several products with 10+ first-filers, leaving ANDA filers to be more selective and shy away from certain products.
More Selective ANDA Filings
The full data set of PIV cases from 2020 is consistent with this observation. Last year, PIV cases were down 15% to 239 PIV cases filed. For perspective, this figure returns the number of PIV cases filed to be in the range the Market witnessed between 2009-2013 (between 199-240 cases per year). By contrast, the average number of PIV cases filed over the past 6 years was 337 per year.
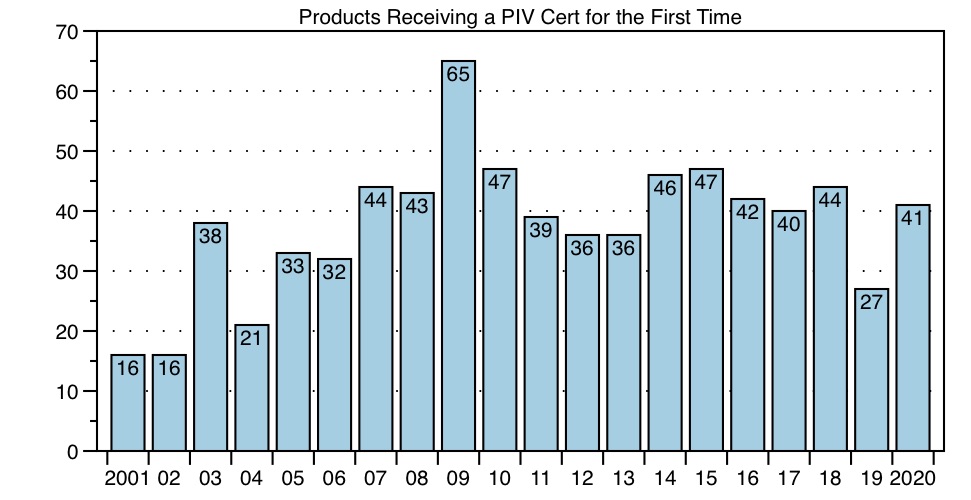
Of course, one reason for fewer filings would be fewer brand products to file against. But the data do not support this possible explanation. On the contrary, as the Graph below illustrates, the number of products receiving their first PIV cases in 2020 increased from last year to 41 products, a number similar to prior years. These data are consistent with the FDA PIV List data — fewer PIV cases yet similar numbers of first-filed products typically means fewer first-filers per product. In fact, in 2020, there were no products that were hypercompetitive (more than 10 filers).
Moving Forward
As the PIV Market moves forward, I think it is pretty safe to conclude that ANDA filers over the past few years have started to file on a select few products, rather than filing on every opportunity. There no reason to believe that this market correction won’t last for a while. PIV Products with 10+ filers is not a sustainable practice or effective strategy. Less is more, in a sense.